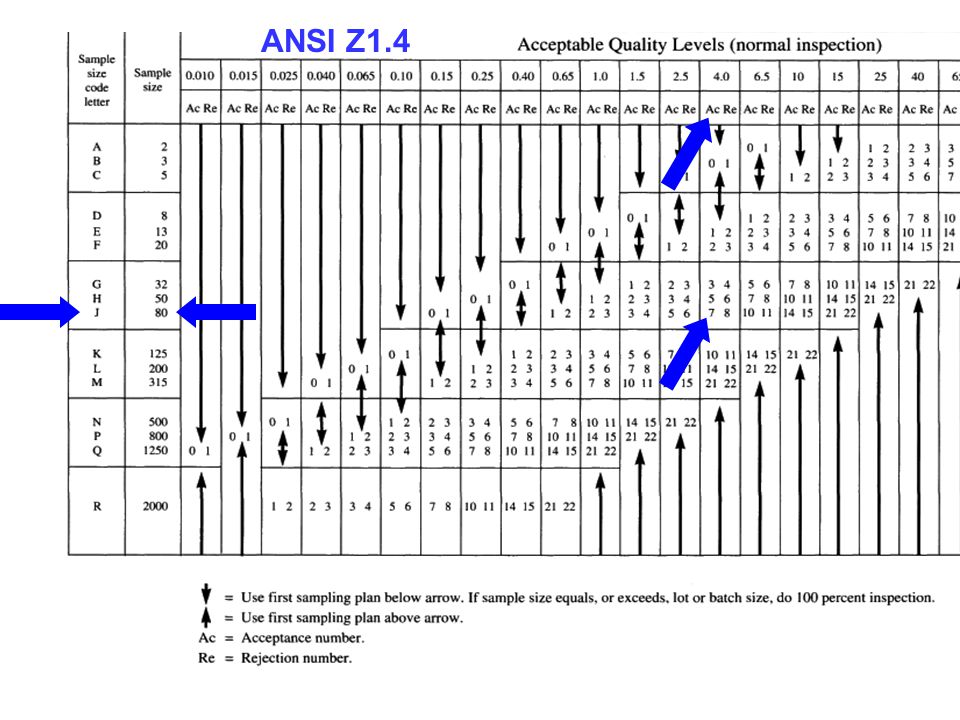
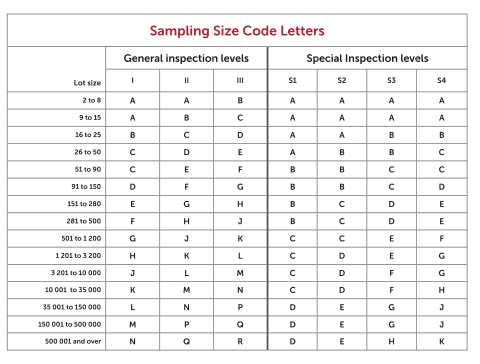
American Society for Quality. ANSI/ASQ Z1.4-Foreword. Iii (This foreword is not a part of the American National. Standard— Sampling Procedures and Tables for Inspection. By Attributes, Z1.4-2003) This standard is a revision of ANSI/ASQC Z1.4-1993, “Sampling Procedures and Tables for Inspection. Attributes.” Beyond editorial refinements. – Notice 1 cancelled the standard and refers DoD users to ANSI/ASQC Z1.4-1993. ANSI/ASQ Z1.4 – Current version is ANSI/ASQ Z1.4: 2008. FDA Recognition – The FDA recognizes ANSI/ASQ Z1.4-2008 as a General consensus standard – Extent of Recognition: Use of all Single, Double and Multiple sampling plans. For a complete explanation of AQL see: The Importer's Guide to Managing Product Quality with AQL C D E E F G S4 A A B C G H C SINGLE SAMPLING PLAN FOR NORMAL INSPECTION, ANSI/ASQ STANDARD Z1.4-2003. Presented on April 25, 2017AbstractAcceptance sampling is a common method to determine if the output of a process, the product, should move to the next proce.


Z1.4–2003 would like to confirm if ASQ Z1. It is a business andi for you to make if your customer is not demanding it. My question is about sampling aluminium foils, films used in packaging and sticker labels received in rolls which are wound around a core. What is the difference between ANSI/ASQC Z1.4 1993 and ANSI/ASQ Z1.4-2003?
|
Ansi Asq Z1 4 2003 R2018
Ansi/asq Z1.4-2003
Ansi/asq Z1.4-2008
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||